When marine algae get sick: how viruses shape microbe interactions
Wake Forest professor takes deeper dive with new study

By looking at the tiniest virus-infected microbes in the ocean, researchers are gaining new insights about the marine food web that may help improve future climate change predictions. The new study, co-authored by Wake Forest Assistant Professor of Biology Sheri Floge, brings together viral ecologists, chemists and physicists to find out more about marine microbes and what happens when viruses infect them.
Photosynthesis in the ocean
On land, photosynthesis is carried out by plants, and the impacts of the process can be seen with the autumn leaves changing color and new green leaves in the spring. But in the ocean, the process is much harder for scientists to measure. Most of the photosynthesis is carried out by the tiniest microbes, specifically phytoplankton. These single-celled organisms also are the base of the marine food web. One of the most abundant types of phytoplankton is marine picocyanobacteria such as Synechococcus.

“Synechococcus are very big players in the ocean globally,” said Sheri Floge, Wake Forest assistant professor of biology. “Together with other picocyanobacteria, they are responsible for about a quarter of ocean photosynthesis and are important to carbon and nutrient cycling, so it’s important that we understand their impacts and how they work, especially as it relates to climate change.”
These microorganisms are susceptible to viral infection and that changes their composition. Until now, scientists didn’t know a lot about what happened when they first became infected. Floge co-authored a new study that takes a deeper dive to demystify this process.
“Historically, scientists have focused on viruses in the ocean for their role in lysing (breaking open) cells. Our work shows that intact virus-infected cells are biochemically and physiologically different from uninfected cells and that other organisms respond differently to them,” said Floge. “There isn’t a lot of research that has focused on the early infection stage. By looking at the infected cells before they lyse, we can learn about their ecological role in ocean communities. And that additional data matters because it gives us a more complete picture of what is happening in marine ecosystems and long-term when you have cyanobacteria-dominated systems in the ocean.”
Collaboration and innovation
Floge partnered with scientists at other institutions, including Tufts University and the Lawrence Berkeley National Laboratory, for the research study.
“When these infected cells die, they release a big patch of food that everything else feeds on,” said Richard J. Henshaw, lead author of the study who served as a postdoc at Tufts University. “But, what we found is that early on, they’re also releasing these strong chemical cues. Other organisms can sense them, and they can swim toward the infected cells, so the early infection process is just as, if not more important, to marine ecosystems as lysis.”
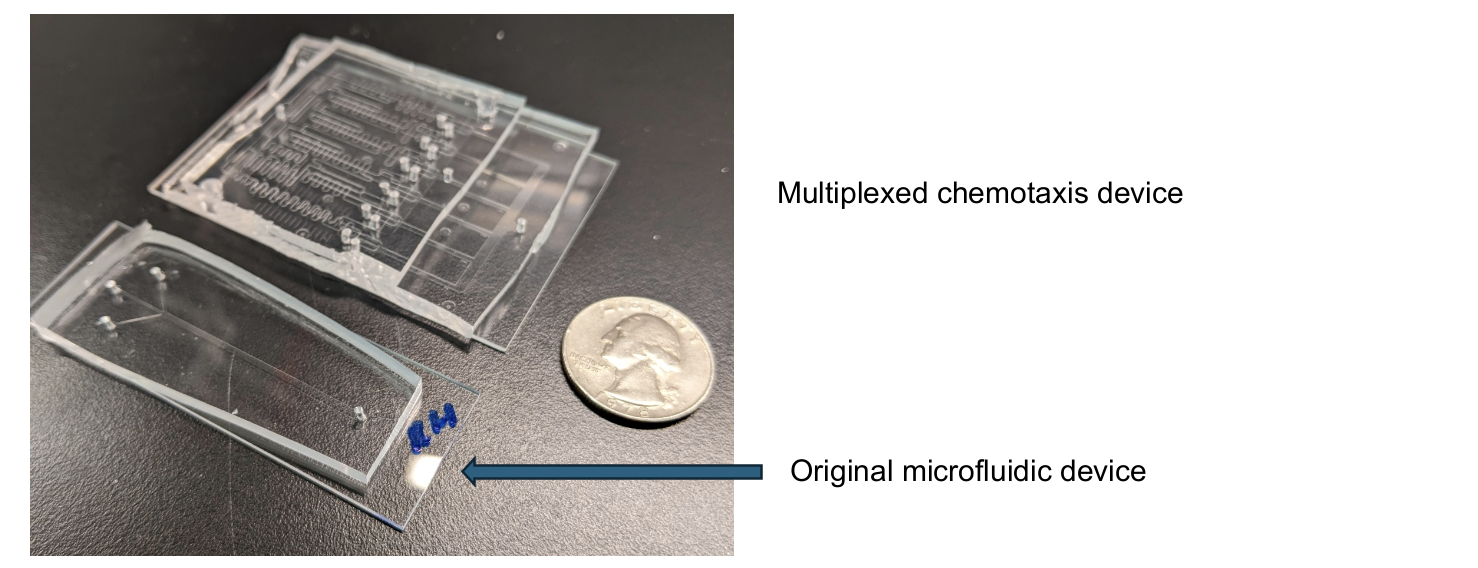
To get a clearer picture of how these microscale interactions work in cells, scientists pushed research techniques to new limits. The paper uses novel techniques (a newly developed multiplexed chemotaxis device) with highly controlled viral infection experiments to better understand the ecological role of viruses and virus-infected microbes in ocean systems.
The new tool was developed by Michael Stehnach, a PhD student at the time at Tufts University and now a postdoc at Brandeis University.
“This innovative device will allow scientists from different fields to rapidly quantify how microbes respond to chemical gradients that have different strengths,” added Henshaw. “So it could be used in industrial and pharmaceuticals for things like research in drug doses or antibiotic resistance. We demonstrated in this paper about phytoplankton and bacteria that using this device is more efficient and can save countless research hours.”
Simulating the ocean environment
Wake Forest undergraduate and graduate students were involved in the research process. Viral infection experiments are carried out in plankton cultures maintained in Wake Forest University’s walk-in growth room lab.
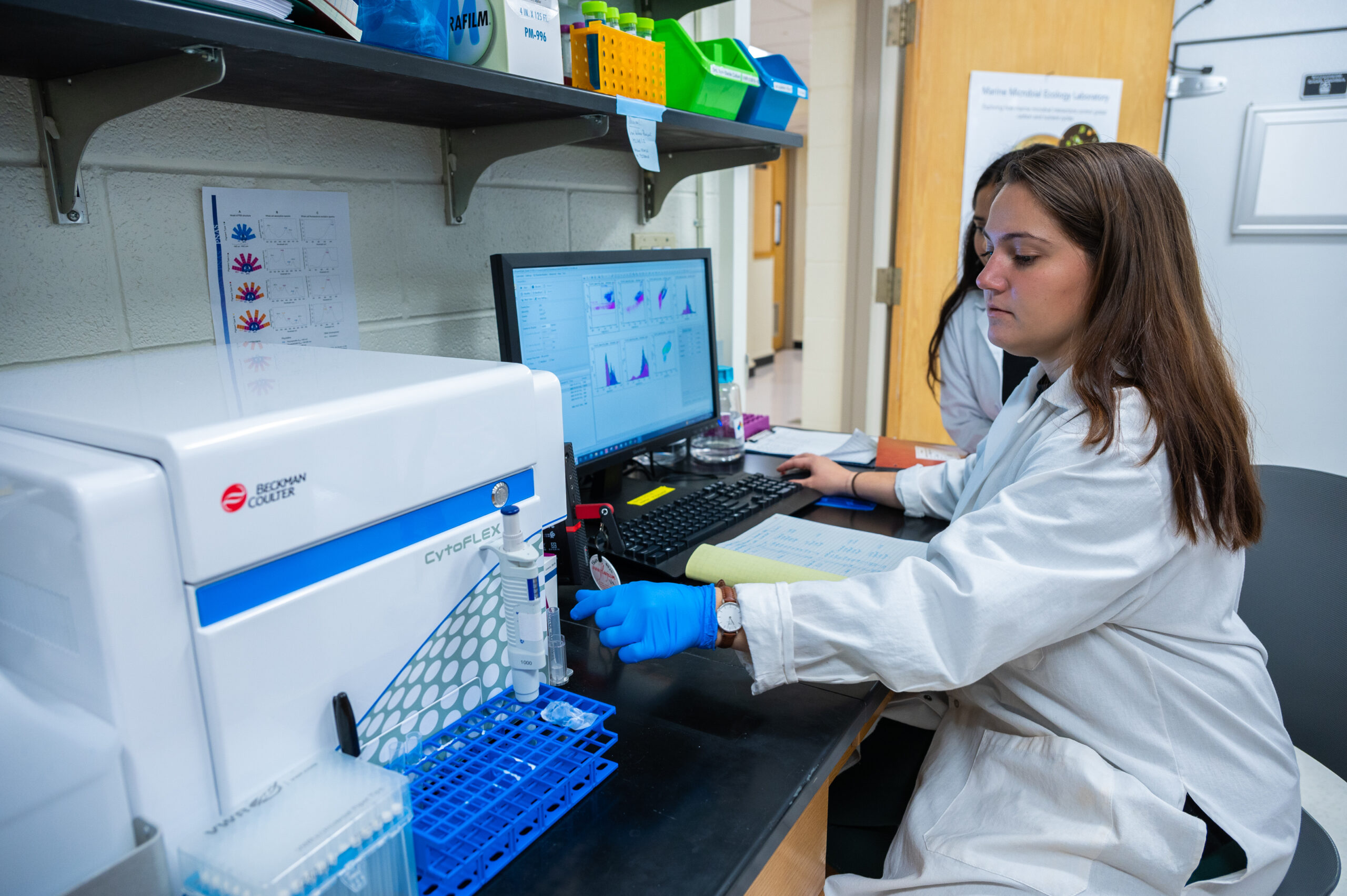
“The walk-in growth room is a highly controlled environment with lighting that can be programmed to simulate natural light conditions,” said Floge. “We run both large- and small-scale viral infection experiments in a range of phytoplankton types grown in artificial seawater to unravel viral impacts on plankton metabolism and interactions.”
Floge said the latest paper builds on her previous research about ocean viruses. She said what she’s most excited about in this new study is how it’s opened a new window for more research.
“When we try to create predictive models of what is going to happen in the future with climate change, a lot of our uncertainty comes back to microbial interactions because they have been very much a black box. This new study helps us unlock the box and gather important data that changes our understanding of some of the roles of viruses in the ocean.”
The study titled: Metabolites from intact phage-infected Synechococcus chemotactically attract heterotrophic marine bacteria” was recently published in Nature Microbiology. DOI: 10.1038/s41564-024-01843-2.
Co-authors also include Jeffrey Guasto (Tufts University) and Trent Northen (Lawrence Berkeley National Laboratory).
Categories: Environment & Sustainability, Experts, Research & Discovery
Media Contact
Keri Brown
media@wfu.edu
336.758.5237



